- Your cart is empty
- Continue Shopping

Product
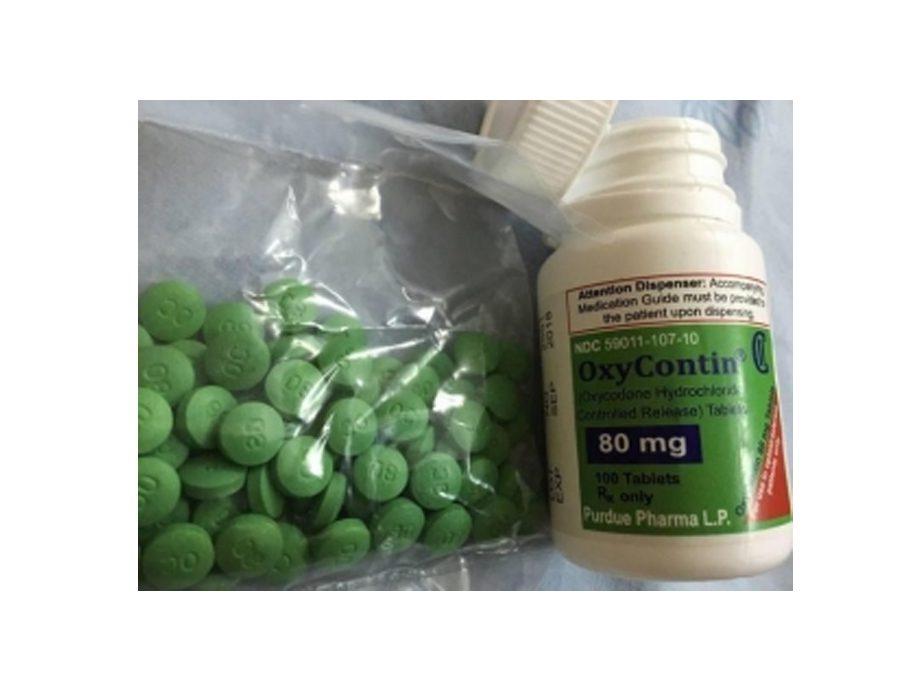
OxyContine 80mg (OXYCODONE HCl CONTROLLED-RELEASE)
$360.00 – $950.00
Pill with imprint OC 80 is Green, Round and has been identified as OxyContin 80 mg. It is supplied by Purdue Pharma LP.
OxyContin is used in the treatment of chronic pain; pain and belongs to the drug class narcotic analgesics. FDA has not classified the drug for risk during pregnancy. OxyContin 80 mg is classified as a Schedule 2 controlled substance under the Controlled Substance Act (CSA)
- Description
- Additional information
- How to take
- Dosage information
- How effective is Oxycodone?
- Side Effects
- Usual Pediatric Dose
- Reviews (0)
Additional Information
| Imprint | OC 80 |
|---|---|
| Strength | 80 mg |
| Color | Green |
| Size | 11.00 mm |
| Shape | Round |
| Quantity | 60 Tabs, 90 Tabs, 120 Tabs, 180 Tabs, 240 Tabs |
How to take
How does Oxycodone work?
Take this medication on a regular schedule as directed by your doctor, not as needed for sudden (breakthrough) pain. Take this drug with or without food, usually every 12 hours. If you have nausea, it may help to take this drug with food. Ask your doctor or pharmacist about other ways to decrease nausea (such as lying down for 1 to 2 hours with as little head movement as possible). If nausea persists, see your doctor.
Dosage information
Adjust dose every 1 to 2 days as needed to obtain an appropriate balance between pain management and opioid-related adverse reactions; goal should be to find the lowest effective dosage for the shortest duration consistent with individual patient treatment goals
Maximum daily dose: Oxycodone (base) ER capsules: 288 mg as the safety of the excipients has not been established; maximum dose of oxycodone hydrochloride tablets has not been established
EQUIVALENCE OF OXYCODONE HYDROCHLORIDE TO OXYCODONE BASE:
-Oxycodone hydrochloride 10 mg = Oxycodone base 9 mg
-Oxycodone hydrochloride 15 mg = Oxycodone base 13.5 mg
-Oxycodone hydrochloride 20 mg = Oxycodone base 18 mg
-Oxycodone hydrochloride 30 mg = Oxycodone base 27 mg
-Oxycodone hydrochloride 40 mg = Oxycodone base 36 mg
How effective is Oxycodone?
-Use of higher starting doses in patients who are not opioid tolerant may cause fatal respiratory depression with first dose; selection of initial dose should take into account degree of opioid tolerance, patient’s general condition, medical status, concurrent medications, type and severity of pain, and risk factors for abuse, addiction, or diversion.
-Opioid tolerant patients are those who have received for 1 week or longer: oral morphine 60 mg/day; transdermal fentanyl 25 mcg/hr; oral oxycodone 30 mg/day; oral hydromorphone 8 mg/day; oral oxymorphone 25 mg/day or an equianalgesic dose of another opioid.
-Extended-release products are reserved for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain; these products are not intended to be used as as-needed (prn) analgesics.
-DOSE CONVERSIONS from other opioids should be done carefully and with close monitoring due to large patient variability in opioid analgesic response; consult dose adjustment section for recommendations.
Use: For the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
Side Effects
What are the side effects?
The US FDA requires a Risk Evaluation and Mitigation Strategy (REMS) for all opioids intended for outpatient use. The new FDA Opioid Analgesic REMS is a designed to assist in communicating the serious risks of opioid pain medications to patients and health care professionals. It includes a medication guide and elements to assure safe use. For additional information: www.accessdata.fda.gov/scripts/cder/rems/index.cfm
US BOXED WARNINGS: RISK OF MEDICATION ERRORS; ADDICTION, ABUSE AND MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; NEONATAL OPIOID WITHDRAWAL SYNDROME; and CYP450 3A4 INTERACTION and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS:
-Risk of Medication Errors: Ensure accuracy when prescribing, dispensing, and administering oxycodone oral solution; dosing errors due to confusion between mg and mL, and other oxycodone oral solutions of different concentrations can result in accidental overdose.
-Addiction, Abuse, and Misuse: Oxycodone exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing and monitor all patients regularly for the development of these behaviors or conditions.
-Opioid Analgesic REMS: To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, a REMS is required for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to complete a REMS-compliant education program; counsel patients and/or their caregivers, with every prescription on safe use, serious risks, storage, and disposal of these products; emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist, and consider other tools to improve patient, household, and community safety.
-Life-Threatening Respiratory Depression: Serious, life-threatening, or fatal respiratory depression may occur. Monitor for respiratory depression, especially during initiation or following a dose increase. Instruct patients to swallow tablets whole; crushing, chewing, or dissolving can cause rapid release and absorption of a potentially fatal dose.
-Accidental Ingestion: Accidental ingestion of even 1 dose of extended-release oxycodone, especially by children, can result in a fatal overdose.
-Neonatal Opioid Withdrawal Syndrome: Prolonged use during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, the patient should be advised of the risk of neonatal opioid withdrawal syndrome and ensure appropriate treatment will be available.
-CYP450 3A4 Interaction: The concomitant use with CYP450 3A4 inhibitors may result in an increase in oxycodone plasma concentrations, which could increase or prolong adverse drug effects and may cause potentially fatal respiratory depression. In addition, discontinuation of a concomitantly used CYP450 3A4 inducer may result in an increase in oxycodone plasma concentration. Monitor patients receiving oxycodone and any CYP450 3A4 inhibitor or inducer.
-Concomitant Use with Benzodiazepines or Other CNS Depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Concomitant use should be reserved for those patients for whom alternative treatment options are inadequate. If needed, limit dose and duration to the minimum required and follow patients for signs and symptoms of respiratory depression and sedation.
Usual Pediatric Dose
Prior to initiating therapy, patients must be receiving and tolerating opioids for at least 5 consecutive days; for the 2 days immediately preceding initiation, patients must be taking a minimum of 20 mg/day of oxycodone or its equivalent.
-Discontinue all around-the-clock opioid drugs when oxycodone ER tablets are initiated
11 years or older: Extended-release (ER) tablets only:
-Initial dose: One-half of calculated total oxycodone daily dose orally every 12 hours
DOSE CALCULATION:
-Multiply total daily dose of prior opioid by the conversion factor (CF) provided below to obtain oxycodone dose in mg/day; divide oxycodone mg/day dose by 2 to get 12-hour oxycodone ER dose; if rounding is necessary, always round down to the nearest tablet strength available
–For prior opioid use of OXYCODONE: Oral CF is 1
–For prior opioid use of HYDROCODONE: Oral CF is 0.9
–For prior opioid use of HYDROMORPHONE: Oral CF is 4; Parenteral CF is 20*
–For prior opioid use of MORPHINE: Oral CF is 0.5; Parenteral CF is 3*
–For prior opioid use of TRAMADOL: Oral CF is 0.17; Parenteral CF is 0.2*
*For patients receiving high-dose parenteral opioids, a more conservative CF is warranted (e.g., for high-dose parenteral morphine, use a CF of 1.5 instead of a CF of 3)
The CFs provided above convert prior opioid use to oxycodone ER tablet dose; do not use the CF to convert from oxycodone ER tablets to another opioid as doing so will result in an over-estimation of the new opioid dose and possibly a fatal overdose.
CONVERSION FROM TRANSDERMAL FENTANYL: Remove patch 18 hours prior to starting oxycodone ER tablets; substitute one 10 mg oxycodone ER tablet every 12 hours for each 25 mcg/hr fentanyl transdermal patch; monitor closely during conversion as there is limited assessment of this conversion
Titration and Maintenance:
-Individually titrate to a dose that provides adequate analgesia and minimizes adverse reactions; dose adjustments can be made every 1 to 2 days; when a dose increase is clinically indicated, it is suggested that the total daily oxycodone dose not be increased by more than 25% at a time.






Reviews
There are no reviews yet.